Carolyn Machamer, Ph.D.
Department of Cell Biology
Johns Hopkins University School of Medicine
725 N. Wolfe Street, 105 WBSB
Baltimore, MD 21205
Academic Titles
ProfessorResearch Topic
Intracellular protein trafficking; enveloped virus assemblyResearch Topic: Golgi complex structure/function; intracellular protein trafficking; intracellular virus assembly; coronaviruses; exocytosis of large cargo
We are interested in the structure and function of the Golgi complex, a ubiquitous eukaryotic organelle that plays a central role in post-translational processing and sorting of newly synthesized proteins and lipids in the secretory pathway. The Golgi complex has an unusual structure, particularly in vertebrates, where stacks of cisternal membranes are clustered into a ribbon structure near the nucleus. One goal of our research is to understand the role of this structure in Golgi function. Towards this goal, we are studying the targeting and function of resident Golgi proteins. We are interested in the contribution of the lipid bilayer to targeting of transmembrane Golgi proteins, and in the function of a group of peripheral Golgi membrane proteins called golgins. Several golgins, including golgin-160, are substrates for caspase cleavage during programmed cell death (apoptosis). Our hypothesis is that specific stress signals are transduced at Golgi membranes, and that cleavage of golgin-160 may be critical for downstream signaling events. In non-apoptotic cells, golgin-160 is involved in trafficking of specific cargo molecules, including the beta-1-adrenergic receptor, and the insulin-regulated glucose transporter, GLUT4.
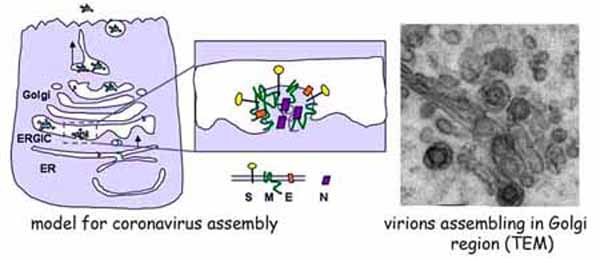
The other research interest in the lab is the assembly mechanism of coronaviruses, enveloped viruses that bud into Golgi compartments. Coronaviruses are vertebrate pathogens that usually cause mild respiratory or gastrointestinal disease. However, the emergence of severe acute respiratory syndrome (SARS), which is caused by a novel coronavirus, has sparked much interest in this group of viruses. We are addressing how coronaviruses target their envelope proteins to Golgi membranes, and how they interact with each other at the virus assembly site. We are also exploring how coronaviruses are exocytosed after they bud into the Golgi lumen. Our long-term goal is to understand the advantages of intracellular assembly for coronaviruses. A better understanding of intracellular assembly and the mechanism of exocytosis should lead to novel strategies for antiviral therapeutics. In addition, we are using coronavirus egress as a model for secretion of large cargo, since the size of the virions results in modification of Golgi structure to allow accommodation of these particles. We hope to learn how other large cargo is trafficked through the Golgi (e.g. chylomicrons in the intestine).