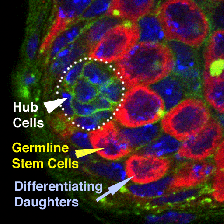
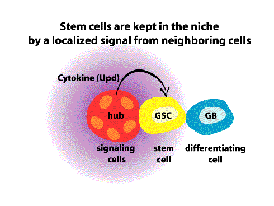
Greenspan, L.J., de Cuevas, M, Le, K.H. Viveiros, J.M. and Matunis, E.L. Activation of the EGFR/MAPK pathway drives transdifferentiation of quiescent niche cells to stem cells in the Drosophila testis Elife. 2022 Apr 25;11:e70810. doi: 10.7554/eLife.70810.PMID: 35468055.
- Hongjie Li, Jasper Janssens, Maxime De Waegeneer, Sai Saroja Kolluru, Kristofer Davie, Vincent Gardeux, Wouter Saelens, Fabrice David, Maria Brbić, Jure Leskovec, Colleen N. McLaughlin, Qijing Xie, Robert C. Jones, Katja Brueckner, Jiwon Shim, Sudhir Gopal Tattikota, Frank Schnorrer, Katja Rust, Todd G. Nystul, Zita Carvalho-Santos, Carlos Ribeiro, Soumitra Pal, Teresa M. Przytycka, Aaron M. Allen, Stephen F. Goodwin, Cameron W. Berry, Margaret T. Fuller, Helen White-Cooper, Erika L. Matunis, Stephen DiNardo, Anthony Galenza, Lucy Erin O’Brien, Julian A. T. Dow, FCA Consortium, Heinrich Jasper, Brian Oliver, Norbert Perrimon, Bart Deplancke, Stephen R. Quake, Liqun Luo, Stein Aerts. Fly Cell Atlas: a single-cell transcriptomic atlas of the adult fruit fly. Science. 2022 Mar 4;375(6584):eabk2432. doi: 10.1126/science.abk2432. Epub 2022 Mar 4. PMID: 35239393.
- Mahadevaraju, S., Fear, J., Akeju, M., Galletta, B., Pinheiro, M., Avelino, C., Cabral-de-Mello, D., Conlon, K., Dell’Orso, S., Demere, Z., Mansuria, K., Mendonca, C., Palacios Giminez, O., Ross, E., Savery, M., Yu, K., Smith, H., Sartorelli V., Nasser, R., Vibranovski, M, Matunis, E. and Oliver, B. Dynamic sex chromosome expression in Drosophila male germ cells. Nat Commun. Feb 9;12(1):892.
Zhang W, Zhang X, Xue Z, Li Y, Ma Q, Ren X, Zhang J, Yang S, Yang L, Wu M, Ren M, Xi R, Wu Z, Liu JL, Matunis E, Dai J, Gao G. (2019). Probing the function of metazoan histones with a systematic library of H3 and H4 mutants. Dev Cell Feb 11;48(3):406-419.
Greenspan, L.J., and Matunis, E.L. (2018). Retinoblastoma intrinsically regulates niche cell quiescence, identity, and niche number in the adult Drosophila testis. Cell Reports 25;24(13):3466-3476.
Greenspan LJ, Matunis EL. 2017. Live Imaging of the Drosophila Testis Stem Cell Niche. Methods Mol Biol. 1463:63-74.
Ma Q, de Cuevas M, Matunis EL. Chinmo is sufficient to induce male fate in somatic cells of the adult Drosophila ovary. Development. 2016 Mar 1;143(5):754-63. doi: 10.1242/dev.129627.
Greenspan LJ, de Cuevas M, Matunis E. Genetics of gonadal stem cell renewal. Annu Rev Cell Dev Biol. 2015;31:291-315. doi: 10.1146/annurev-cellbio-100913-013344.
Hasan, S., Hétié, P., and Matunis, E.L. (2015). Niche signaling promotes stem cell survival in the Drosophila testis via the JAK-STAT target DIAP1. Dev Biol 404, 27-39.
Stine, R.R., Greenspan, L.J., Ramachandran, K.V., and Matunis, E.L. (2014). Coordinate regulation of stem cell competition by Slit-Robo and JAK-STAT signaling in the Drosophila testis. PLoS Genet 10, e1004713.
- Ma, Q., Wawersik, M., Matunis, EL. 2014. The Jak-STAT target Chinmo prevents sex transformation of adult stem cells in the Drosophila testis niche. Dev Cell 31, 474-486.
Li, Y., Ma, Q., Cherry, C.M., and Matunis, E.L. (2014). Steroid signaling promotes stem cell maintenance in the Drosophila testis. Dev Biol 394, 129-141.
Hetie, P., de Cuevas, M. and Matunis, E. 2014. Conversion of Quiescent Niche Cells to Somatic Stem Cells Causes Ectopic Niche Formation in the Drosophila Testis. Cell Reports 7, 715-721.
Stine RR, Matunis EL. Stem cell competition: finding balance in the niche. Trends Cell Biol. 2013 Aug;23(8):357-64.
Matunis EL, Stine RR, de Cuevas M. 2012. Recent advances in Drosophila male germline stem cell biology. Spermatogenesis 2: 137-144. PMID: 23087833.
Sinden D, Badgett M, Fry J, Jones T, Palmen R, Sheng X, Simmons A, Matunis E, Wawersik M. 2012. Jak-STAT regulation of cyst stem cell development in the Drosophila testis. Dev. Biol. 372: 5-16. PMID: 23010510.
Issigonis M, Matunis E. 2012. The Drosophila BCL6 homolog Ken and Barbie promotes somatic stem cell self-renewal in the testis niche. Dev. Biol. 368: 181-92. PMID: 22580161.
Sheng, X. R., Matunis, E. L. 2011. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development 16:3367-3376.
de Cuevas, M., Matunis, E. L. 2011. The stem cell niche: Lessons from the Drosophila testis. Development 14:2861-2969.
Cherry, C.M., Matunis, E.L. 2010. Epigenetic regulation of stem cell maintenance in the Drosophila testis via the nucleosome remodeling factor NURF. Cell Stem Cell 6:557-567.
Issigonis, M., Tulina, N., de Cuevas, M., Brawley, C., Sandler, L., Matunis, E. 2009. JAK-STAT signal inhibition regulates competition within the Drosophila testis stem cell niche. Science 326:153-6.
Sheng, X.R., Brawley, C.M., Matunis, E.L. 2009. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell 5:191-203.
Sheng, X.R., Posenau, T., Gumulak-Smith, J.J., Matunis, E., Van Doren, M., Wawersik, M., 2009. Jak-STAT regulation of male germline stem cell establishment during Drosophila embryogenesis. Dev. Biol. 334:335-344.
Buszczak, M., Paterno, S., Lighthouse D., Bachman J., Plank J., Owen S., Skora A., Nystul T., Ohlstein B., Allen A., Wilhelm J., Murphy T., Levis B., Matunis. E., Srivali N., Hoskins R., Spradling A. 2007. The Carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics 175:1501-1531.
Terry, N.A, Tulina, N., Matunis, E., DiNardo S. 2006. Novel regulators revealed by profiling Drosophila testis stem cells within their niche. Dev. Biol. 294:246-257.
Wawersik, M., Milutinovich, A. Matunis, E., Williams, B. Van Doren, M. 2005. Somatic control of germline sexual differentiation is mediated by the JAK/STAT pathway. Nature 436:563-567.
Brawley, C, Matunis, E. 2004. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 304:1331-1334.
Tulina, N., Matunis, E. 2001. Control of Stem cell self-renewal in Drosophila spermatogenesis by Jak-STAT signaling. Science 294: 2546-2549.